
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Drug/Regimen
- Efficacy and Safety of IDegAsp in a Real-World Korean Population with Type 2 Diabetes Mellitus
- Shinae Kang, Yu-Bae Ahn, Tae Keun Oh, Won-Young Lee, Sung Wan Chun, Boram Bae, Amine Dahaoui, Jin Sook Jeong, Sungeun Jung, Hak Chul Jang
- Received August 24, 2023 Accepted November 22, 2023 Published online February 27, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0297 [Epub ahead of print]
- 671 View
- 47 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
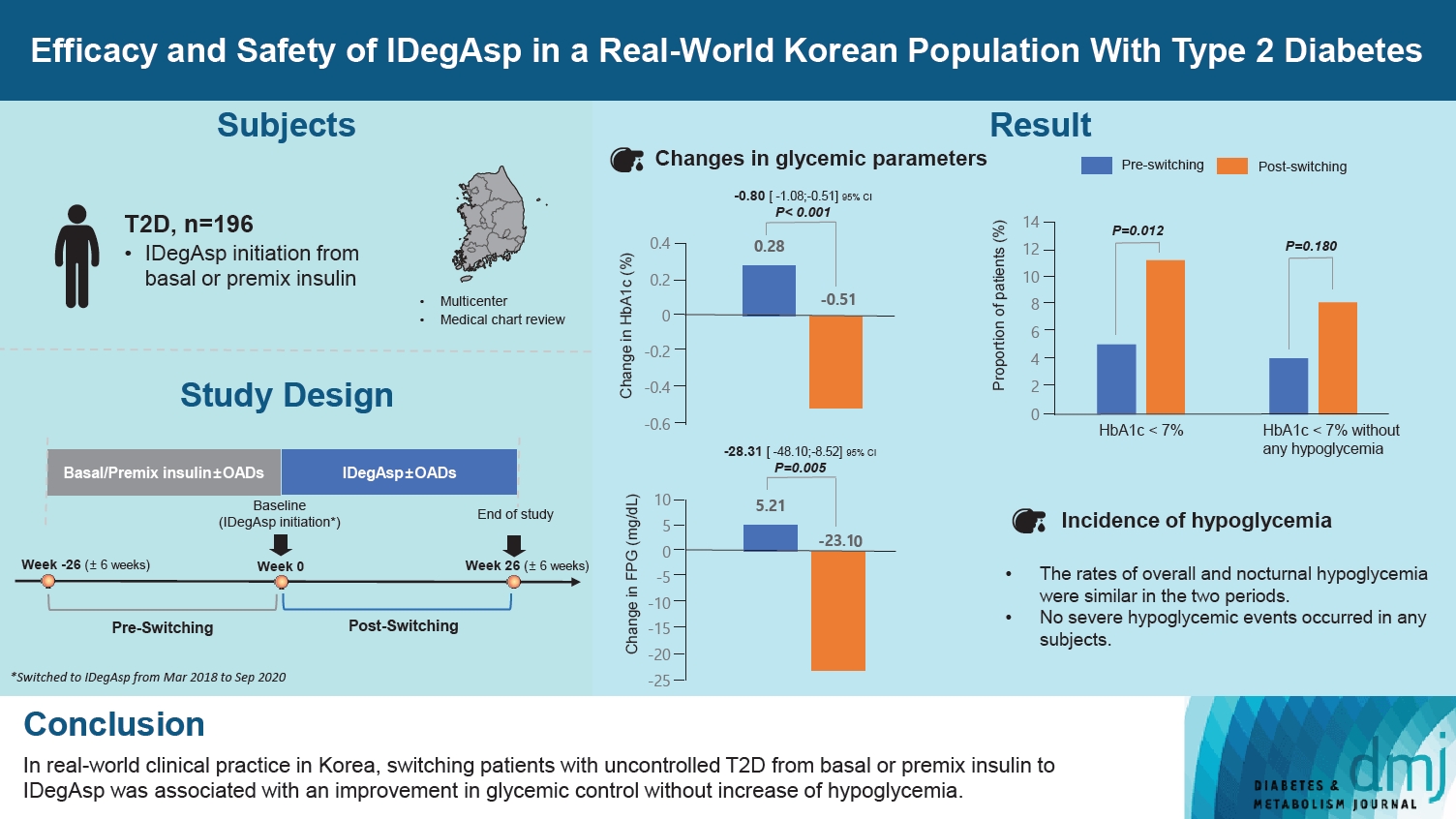
This study investigated the real-world efficacy and safety of insulin degludec/insulin aspart (IDegAsp) in Korean adults with type 2 diabetes mellitus (T2DM), whose insulin treatment was switched to IDegAsp.
Methods
This was a multicenter, retrospective, observational study comprising two 26-week treatment periods, before and after switching to IDegAsp, respectively. Korean adults with uncontrolled T2DM treated with basal or premix insulin (±oral antidiabetic drugs) were enrolled. The primary objective was to compare the degree of glycosylated hemoglobin (HbA1c) change in each 26-week observation period. The analyses included changes in HbA1c, fasting plasma glucose (FPG), body weight, proportion of participants achieving HbA1c <7.0%, hypoglycemic events, and total daily insulin dose (ClinicalTrials.gov, number NCT04656106).
Results
In total, 196 adults (mean age, 65.95 years; mean T2DM duration, 18.99 years) were analyzed. The change in both HbA1c and FPG were significantly different between the pre-switching and the post-switching period (0.28% vs. –0.51%, P<0.001; 5.21 mg/dL vs. –23.10 mg/dL, P=0.005), respectively. After switching, the rate of achieving HbA1c <7.0% was significantly improved (5.10% at baseline vs. 11.22% with IDegAsp, P=0.012). No significant differences (before vs. after switching) were observed in body weight change, and total daily insulin dose. The rates of overall and severe hypoglycemia were similar in the two periods.
Conclusion
In real-world clinical practice in Korea, the change of insulin regimen to IDegAsp was associated with an improvement in glycemic control without increase of hypoglycemia, supporting the use of IDegAsp for patients with T2DM uncontrolled with basal or premix insulin.
- Safety and Efficacy of Modern Insulin Analogues
- Hye Jin Yoo, Keun Yong Park, Kang Seo Park, Kyu Jeung Ahn, Kyung Wan Min, Jeong Hyun Park, Sang Ah Chang, Bong Soo Cha, Dong-Jun Kim, Yong Seong Kim, Tae Keun Oh, Suk Chon, Il Seong Nam-Goong, Mi Jin Kim, Hye-Soon Kim, Young Sik Choi, You Hern Ahn, Sora Lee, Sei Hyun Baik
- Diabetes Metab J. 2013;37(3):181-189. Published online June 14, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.3.181
- 4,131 View
- 32 Download
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background A1chieve® was a noninterventional study evaluating the clinical safety and efficacy of biphasic insulin aspart 30, insulin detemir, and insulin aspart.
Methods Korean type 2 diabetes patients who have not been treated with the study insulin or have started it within 4 weeks before enrollment were eligible for the study. The patient selection and the choice of regimen were at the discretion of the physician. The safety and efficacy information was collected from the subjects at baseline, week 12, and week 24. The number of serious adverse drug reactions (SADRs) was the primary endpoint. The changes of clinical diabetic markers at week 12 and/or at week 24 compared to baseline were the secondary endpoints.
Results Out of 4,058 exposed patients, 3,003 completed the study. During the study period, three SADRs were reported in three patients (0.1%). No major hypoglycemic episodes were observed and the rate of minor hypoglycemic episodes marginally decreased during 24 weeks (from 2.77 to 2.42 events per patient-year). The overall quality of life score improved (from 66.7±15.9 to 72.5±13.5) while the mean body weight was slightly increased (0.6±3.0 kg). The 24-week reductions in glycated hemoglobin, fasting plasma glucose and postprandial plasma glucose were 1.6%±2.2%, 2.5±4.7 mmol/L, and 4.0±6.4 mmol/L, respectively.
Conclusion The studied regimens showed improvements in glycemic control with low incidence of SADRs, including no incidence of major hypoglycemic episodes in Korean patients with type 2 diabetes.
-
Citations
Citations to this article as recorded by- Insulin therapy for adult patients with type 2 diabetes mellitus: a position statement of the Korean Diabetes Association, 2017
Byung-Wan Lee, Jin Hwa Kim, Seung-Hyun Ko, Kyu Yeon Hur, Nan-Hee Kim, Sang Youl Rhee, Hyun Jin Kim, Min Kyong Moon, Seok-O Park, Kyung Mook Choi
The Korean Journal of Internal Medicine.2017; 32(6): 967. CrossRef - Insulin Therapy for Adult Patients with Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association, 2017
Byung-Wan Lee, Jin Hwa Kim, Seung-Hyun Ko, Kyu-Yeon Hur, Nan-Hee Kim, Sang Youl Rhee, Hyun Jin Kim, Min Kyong Moon, Seok-O Park, Kyung Mook Choi
Diabetes & Metabolism Journal.2017; 41(5): 367. CrossRef - An information and communication technology-based centralized clinical trial to determine the efficacy and safety of insulin dose adjustment education based on a smartphone personal health record application: a randomized controlled trial
Gyuri Kim, Ji Cheol Bae, Byoung Kee Yi, Kyu Yeon Hur, Dong Kyung Chang, Moon-Kyu Lee, Jae Hyeon Kim, Sang-Man Jin
BMC Medical Informatics and Decision Making.2017;[Epub] CrossRef - Characteristics Predictive for a Successful Switch from Insulin Analogue Therapy to Oral Hypoglycemic Agents in Patients with Type 2 Diabetes
Gyuri Kim, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Yonsei Medical Journal.2016; 57(6): 1395. CrossRef - Avoiding or coping with severe hypoglycemia in patients with type 2 diabetes
Jae-Seung Yun, Seung-Hyun Ko
The Korean Journal of Internal Medicine.2015; 30(1): 6. CrossRef - Clinical Characteristics of Patients Responding to Once-Daily Basal Insulin Therapy in Korean Subjects with Type 2 Diabetes
Sun Ok Song, You-Cheol Hwang, Kyu-Jeung Ahn, Bong Soo Cha, Young Duk Song, Dae Wook Lee, Byung-Wan Lee
Diabetes Therapy.2015; 6(4): 547. CrossRef - The optimal morning:evening ratio in total dose of twice‐daily biphasic insulin analogue in poorly controlled Type 2 diabetes: a 24‐week multi‐centre prospective, randomized controlled, open‐labelled clinical study
C. H. Jung, J.‐Y. Park, J. H. Cho, K.‐H. Yoon, H. K. Yang, Y.‐H. Lee, B. S. Cha, B.‐W. Lee
Diabetic Medicine.2014; 31(1): 68. CrossRef -
The glycemic efficacies of insulin analogue regimens according to baseline glycemic status in Korean patients with type 2 diabetes: sub‐analysis from the A
1
chieve
®
study
Y.‐C. Hwang, J. G. Kang, K. J. Ahn, B. S. Cha, S.‐H. Ihm, S. Lee, M. Kim, B.‐W. Lee
International Journal of Clinical Practice.2014; 68(11): 1338. CrossRef - Letter: Efficacy and Safety of Biphasic Insulin Aspart 30/70 in Type 2 Diabetes Suboptimally Controlled on Oral Antidiabetic Therapy in Korea: A Multicenter, Open-Label, Single-Arm Study (Diabetes Metab J2013;37:117-24)
Byung-Wan Lee
Diabetes & Metabolism Journal.2013; 37(3): 212. CrossRef
- Insulin therapy for adult patients with type 2 diabetes mellitus: a position statement of the Korean Diabetes Association, 2017
- Comparison of Vildagliptin-Metformin and Glimepiride-Metformin Treatments in Type 2 Diabetic Patients
- Hyun Jeong Jeon, Tae Keun Oh
- Diabetes Metab J. 2011;35(5):529-535. Published online October 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.5.529
- 65,535 View
- 98 Download
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The present study investigated the efficacy and safety of vildagliptin-metformin treatment compared to those of glimepiride-metformin treatment for type 2 diabetes.
Methods In a randomized, open-label, comparative study, 106 patients with type 2 diabetes were enrolled. The primary endpoint was a reduction in HbA1c from baseline and secondary endpoints included fasting plasma glucose (FPG) or 2-hour postprandial glucose (2h-PPG) reduction from baseline, as well as HbA1c responder rate and HbA1c reduction according to baseline HbA1c category.
Results Comparable HbA1c reduction was observed with a mean±standard deviation change from baseline to the 32-week endpoint of -0.94±1.15% in the vildagliptin group and -1.00±1.32% in the glimepiride group. A similar reduction in 2h-PPG (vildagliptin group 3.53±4.11 mmol/L vs. the glimepiride group 3.72±4.17 mmol/L) was demonstrated, and the decrements in FPG (vildagliptin group 1.54±2.41 mmol/L vs. glimepiride group 2.16±2.51 mmol/L) were not different between groups. The proportion of patients who achieved an HbA1c less than 7% at week 32 was 50.1% in the vildagliptin group and 56.0% in the glimepiride group. An average body weight gain of 2.53±1.21 kg in the glimepiride group was observed in contrast with the 0.23±0.69 kg weight gain noted in the vildagliptin group. A 10-fold lower incidence of hypoglycemia was demonstrated in the vildagliptin group, in addition to an absence of severe hypoglycemia.
Conclusion Vildagliptin-metformin treatment provided blood glucose control efficacy comparable to that of glimepiride-metformin treatment and resulted in better adverse event profiles with lower risks of hypoglycemia and weight gain.
-
Citations
Citations to this article as recorded by- A Randomized, Two-Treatments, Two-Periods, Crossover, Open label, Laboratory-Blind, Single Dose Bioequivalence Study between Vildagliptin/Metformin 50 mg/1000 mg Film Coated Tablets (Sensityn®) and Galvusmet® 50 mg/1000 mg Film Coated Tablets in healthy a
J. Shiekmydeen, T. Siddiqi, K. Chakraborty, S. Khalaf, M. Albarazi, I. Eqtefan, J. Sliva
European Pharmaceutical Journal.2023; 70(2): 1. CrossRef - Bioequivalence Studies of New Generic Formulations of Vildagliptin and Fixed-Drug Combination of Vildagliptin and Metformin Versus Respective Originator Products in Healthy Volunteers
Yvonne Schnaars, Sumedh Gaikwad, Ulrike Gottwald-Hostalek, Ulrike Klingberg, Hari Kiran Chary Vadla, Vamshi Ramana Prathap
Diabetes Therapy.2022; 13(6): 1215. CrossRef - Efficacy and safety of dorzagliatin for type 2 diabetes mellitus: A meta-analysis and trial sequential analysis
Yunfeng Yu, Xingyu Yang, Keke Tong, Shuang Yin, Gang Hu, Fei Zhang, Pengfei Jiang, Manli Zhou, Weixiong Jian
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - A Single-Center, Observational, Retrospective Cost-Effective Analysis of Treating Inadequately Controlled Type 2 Diabetes Mellitus by Addition of DPP4 Inhibitors Versus Intensified Treatment with Conventional Drugs
Akshata Kalyani, Sachin Kuchya, >Prashant Punekar
Journal of Pharmacology and Pharmacotherapeutics.2021; 12(3): 125. CrossRef - Comparison of safety and efficacy of glimepiride-metformin and vildagliptin- metformin treatment in newly diagnosed type 2 diabetic patients
Surendra Kumar
Indian Journal of Endocrinology and Metabolism.2021; 25(4): 326. CrossRef - Comparative clinical study evaluating the effect of adding Vildagliptin versus Glimepiride to ongoing Metformin therapy on diabetic patients with symptomatic coronary artery disease
Rehab Werida, Mahmoud Kabel, Gamal Omran, Ahmed Shokry, Tarek Mostafa
Diabetes Research and Clinical Practice.2020; 170: 108473. CrossRef - Efficacy of different antidiabetic drugs based on metformin in the treatment of type 2 diabetes mellitus: A network meta‐analysis involving eight eligible randomized‐controlled trials
Yan Peng, Shu‐Hong Chen, Xiao‐Nan Liu, Qing‐Yun Sun
Journal of Cellular Physiology.2019; 234(3): 2795. CrossRef - A safety and tolerability profile comparison between dipeptidyl peptidase-4 inhibitors and sulfonylureas in diabetic patients: A systematic review and meta-analysis
Daniela Farah, Graziella Malzoni Leme, Freddy Goldberg Eliaschewitz, Marcelo Cunio Machado Fonseca
Diabetes Research and Clinical Practice.2019; 149: 47. CrossRef - Efficacy and safety of dulaglutide monotherapy compared with glimepiride in East‐Asian patients with type 2 diabetes in a multicentre, double‐blind, randomized, parallel‐arm, active comparator, phase III trial
Yu Hong Chen, Chien‐Ning Huang, Young Min Cho, Pengfei Li, Liqun Gu, Feng Wang, Jun Yang, Wei Qing Wang
Diabetes, Obesity and Metabolism.2018; 20(9): 2121. CrossRef - Cost effectiveness of vildagliptin versus glimepiride as add-on treatment to metformin for the treatment of diabetes mellitus type 2 patients in Greece
Hara Kousoulakou, Magdalini Hatzikou, Varvara Baroutsou, John Yfantopoulos
Cost Effectiveness and Resource Allocation.2017;[Epub] CrossRef - The efficacy and safety of adding either vildagliptin or glimepiride to ongoing metformin therapy in patients with type 2 diabetes mellitus
Gyuri Kim, Sewon Oh, Sang-Man Jin, Kyu Yeon Hur, Jae Hyeon Kim, Moon-Kyu Lee
Expert Opinion on Pharmacotherapy.2017; 18(12): 1179. CrossRef - Predictors of efficacy of GLP-1 agonists and DPP-4 inhibitors: A systematic review
Helene Bihan, Winda L. Ng, Dianna J. Magliano, Jonathan E. Shaw
Diabetes Research and Clinical Practice.2016; 121: 27. CrossRef - New Oral Diabetes Drugs are more effective than Older Agents: Real or a Fraud?
Udaya M Kabadi
Journal of Diabetes, Metabolic Disorders & Control.2016;[Epub] CrossRef - Systematic review and meta-analysis of vildagliptin for treatment of type 2 diabetes
Eleni Bekiari, Chrysoula Rizava, Eleni Athanasiadou, Konstantinos Papatheodorou, Aris Liakos, Thomas Karagiannis, Maria Mainou, Maria Rika, Panagiota Boura, Apostolos Tsapas
Endocrine.2016; 52(3): 458. CrossRef - Sulfonylurea Glimepiride: A Proven Cost Effective, Safe and Reliable War Horse in Combating Hyperglycemia in Type 2 Diabetes
Udaya M. Kabadi
Journal of Diabetes Mellitus.2015; 05(04): 211. CrossRef - Glycemic effects of vildagliptin and metformin combination therapy in Indian patients with type 2 diabetes: An observational study (印度2型糖尿病患者使用维格列汀与二甲双胍联合治疗对血糖的影响:一项观察性研究)
Sanjay Chatterjee, Sudip Chatterjee
Journal of Diabetes.2014; 6(3): 237. CrossRef - Head‐to‐head comparison of dipeptidyl peptidase‐IV inhibitors and sulfonylureas – a meta‐analysis from randomized clinical trials
Yifei Zhang, Jie Hong, Jie Chi, Weiqiong Gu, Guang Ning, Weiqing Wang
Diabetes/Metabolism Research and Reviews.2014; 30(3): 241. CrossRef - Vildagliptin: A Review of Its Use in Type 2 Diabetes Mellitus
Gillian M. Keating
Drugs.2014; 74(5): 587. CrossRef - Vildagliptin compared to glimepiride on post-prandial lipemia and on insulin resistance in type 2 diabetic patients
Giuseppe Derosa, Aldo Bonaventura, Lucio Bianchi, Davide Romano, Elena Fogari, Angela D’Angelo, Pamela Maffioli
Metabolism.2014; 63(7): 957. CrossRef - The Placement of DPP-4 Inhibitors in Clinical Practice Recommendations for the Treatment of Types 2 Diabetes
Jaime A. Davidson
Endocrine Practice.2013; 19(6): 1050. CrossRef - Predictive Clinical Parameters and Glycemic Efficacy of Vildagliptin Treatment in Korean Subjects with Type 2 Diabetes
Jin-Sun Chang, Juyoung Shin, Hun-Sung Kim, Kyung-Hee Kim, Jeong-Ah Shin, Kun-Ho Yoon, Bong-Yun Cha, Ho-Young Son, Jae-Hyoung Cho
Diabetes & Metabolism Journal.2013; 37(1): 72. CrossRef - The Efficacy of Vildagliptin in Korean Patients with Type 2 Diabetes
Jun Sung Moon, Kyu Chang Won
Diabetes & Metabolism Journal.2013; 37(1): 36. CrossRef - Effect of Vildagliptin on Glucose and Insulin Concentrations During a 24-Hour Period in Type 2 Diabetes Patients with Different Ranges of Baseline Hemoglobin A1c Levels
Manuel González-Ortiz, María J. Sánchez-Peña, Luis J. González-Ortiz, José A. Robles-Cervantes, Yessica E. García-Ortega, Esteban A. Gómez-Gaitán, Karina G. Pérez-Rubio, Esperanza Martínez-Abundis
Diabetes Technology & Therapeutics.2013; 15(7): 564. CrossRef - Differential effects of vildagliptin and glimepiride on glucose fluctuations in patients with type 2 diabetes mellitus assessed using continuous glucose monitoring
Y. L. He, G. Foteinos, S. Neelakantham, D. Mattapalli, K. Kulmatycki, T. Forst, A. Taylor
Diabetes, Obesity and Metabolism.2013; 15(12): 1111. CrossRef - Combination therapy with metformin plus vildagliptin in type 2 diabetes mellitus
Elisa Guarino, Laura Nigi, Aurora Patti, Cecilia Fondelli, Francesco Dotta
Expert Opinion on Pharmacotherapy.2012; 13(9): 1377. CrossRef
- A Randomized, Two-Treatments, Two-Periods, Crossover, Open label, Laboratory-Blind, Single Dose Bioequivalence Study between Vildagliptin/Metformin 50 mg/1000 mg Film Coated Tablets (Sensityn®) and Galvusmet® 50 mg/1000 mg Film Coated Tablets in healthy a
- Association Study of the Peroxisome Proliferators-Activated Receptor gamma2 Pro12Ala Polymorphism with Diabetic Nephropathy.
- Kyu Ho Lee, Hee Seog Jeong, Khan Young Choi, Hyun Kim, Dal Sic Lee, Ji Young Kang, Hyun Jeong Jeon, Tae Keun Oh
- Korean Diabetes J. 2008;32(5):402-408. Published online October 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.5.402
- 1,913 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Peroxisome proliferators-activated receptor gamma (PPARgamma) is a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors and known to play a role in regulating the expression of numerous genes involved in lipid metabolism, metabolic syndrome, inflammation, and atherosclerosis. The PPARgamma2 Pro12Ala polymorphism has recently been shown to be associated with diabetic nephropathy. In this study, we evaluated the relationship between PPARgamma2 Pro12Ala polymorphism and type 2 diabetic nephropathy whose duration of diabetes was over 10 years. METHODS: We conducted a case-control study, which enrolled 367 patients with type 2 diabetes. Genotyping of PPARgamma2 Pro12Ala polymorphism was performed using polymerase chain reaction followed by digestion with Hae III restriction enzyme. RESULTS: The genotype or allele frequencies of PPARgamma2 Pro12Ala polymorphism were not significantly different in diabetic patients with or without diabetic nephropathy. The genotype frequencies in terms of diabetic retinopathy and macrovascular complications such as coronary artery disease or stroke were not different either. Interestingly, nephropathy patients with Ala/Pro genotype showed lower C-peptide levels than those of Pro/Pro genotype. CONCLUSION: Our results suggest that PPARgamma2 Pro12Ala polymorphism is not associated with diabetic nephropathy in type 2 diabetic patients.
- Transforming Growth Factor-beta 1 Gene Polymorphisms According to Diabetic Nephropathy in Type 2 Diabetes.
- Hyun Jeong Jeon, Ok Hee Kim, Kil Ho, Soon Kil Kwon, Tae Keun Oh
- Korean Diabetes J. 2007;31(2):144-150. Published online March 1, 2007
- DOI: https://doi.org/10.4093/jkda.2007.31.2.144
- 1,417 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Transforming growth factor-beta is known to play a role in the interaction between metabolic and hemodynamic factors in mediating accumulation of extracellular matrix in the diabetic nephropathy. TGF-beta1 gene polymorphism was associated with circulating TGF-beta levels and influenced the pathogenesis of fibrotic diseases including diabetic nephropathy. In this study, we examined the relationship between TGF-beta1 gene codon 10 polymorphism and type 2 diabetic nephropathy with more than 10-year history of disease. METHODS: We conducted a case-control study, which enrolled 325 type 2 diabetes. A total of 176 patients with diabetic nephropathy were compared with 149 patients without diabetic nephropathy. TGF-beta1 codon 10 genotyping was determined using polymerase chain reaction with sequence specific primers method. RESULTS: Distribution of TGF-beta1 codon 10 genotype in the patients either with nephropathy or without nephropathy is confined to Hardy-Weinberg equilibrium. The patients with nephropathy have higher frequency of TGF-beta1 GA/GG genotypes than the patients without nephropathy [GA/GG:AA = 119 (67.6%) : 57 (32.4%) vs. 80 (53.7%) : 69 (46.3%), P < 0.05]. Among patients with diabetic nephropathy, those with TGF-beta1 GA/GG genotypes had higher serum levels of total cholesterol and LDL-cholesterol. CONCLUSION: Our results suggest that TGF-beta1 gene codon 10 polymorphism may contribute to the type 2 diabetic nephropathy.

 KDA
KDA
 First
First Prev
Prev





